Pharmaceutical sector
Electrical systems for pharmaceutical facilities with CQV validation
There are numerous stakeholders involved in the pharmaceutical facilities sector: designers, general contractors, safety technicians, and many others. It's essential to consider all the protocols and procedures to make the facility compliant and in accordance with Good Manufacturing Practice (GMP) regulations.
What makes us unique is our ability to provide a 360-degree approach. We can handle the design, as well as the project evaluation process, to ensure it meets all the quality standards and regulations necessary for commissioning.
Quality is our fundamental pillar, and with our extensive experience and expertise, we work closely with highly specialized companies.
We tackle every challenge with dedication and flexibility, adapting to our clients' needs and the evolving regulatory landscape of the industry.
With decades of experience in the field, we can simplify documents and procedures, guiding you professionally and competently through every phase.

Design, installation, and maintenance
of customized electrical systems
for pharmaceutical facilities.

Medium voltage and low voltage systems
Our company specializes in the design and commissioning of Medium Voltage and Low Voltage systems.
Moreover, we offer testing and commissioning services for MT switchgear, transformers, power centers, and BT lines.
Our experience and expertise enable us to provide customized and high-quality solutions tailored to the specific needs of our pharmaceutical sector clients, ensuring the safe and efficient operation of electrical systems.
Instrumental design
Thanks to specific expertise in instrumental design, we optimize the efficiency and safety of pharmaceutical facilities, providing tailored solutions for production needs.
We carefully assess processes and provide targeted optimizations to maximize productivity.
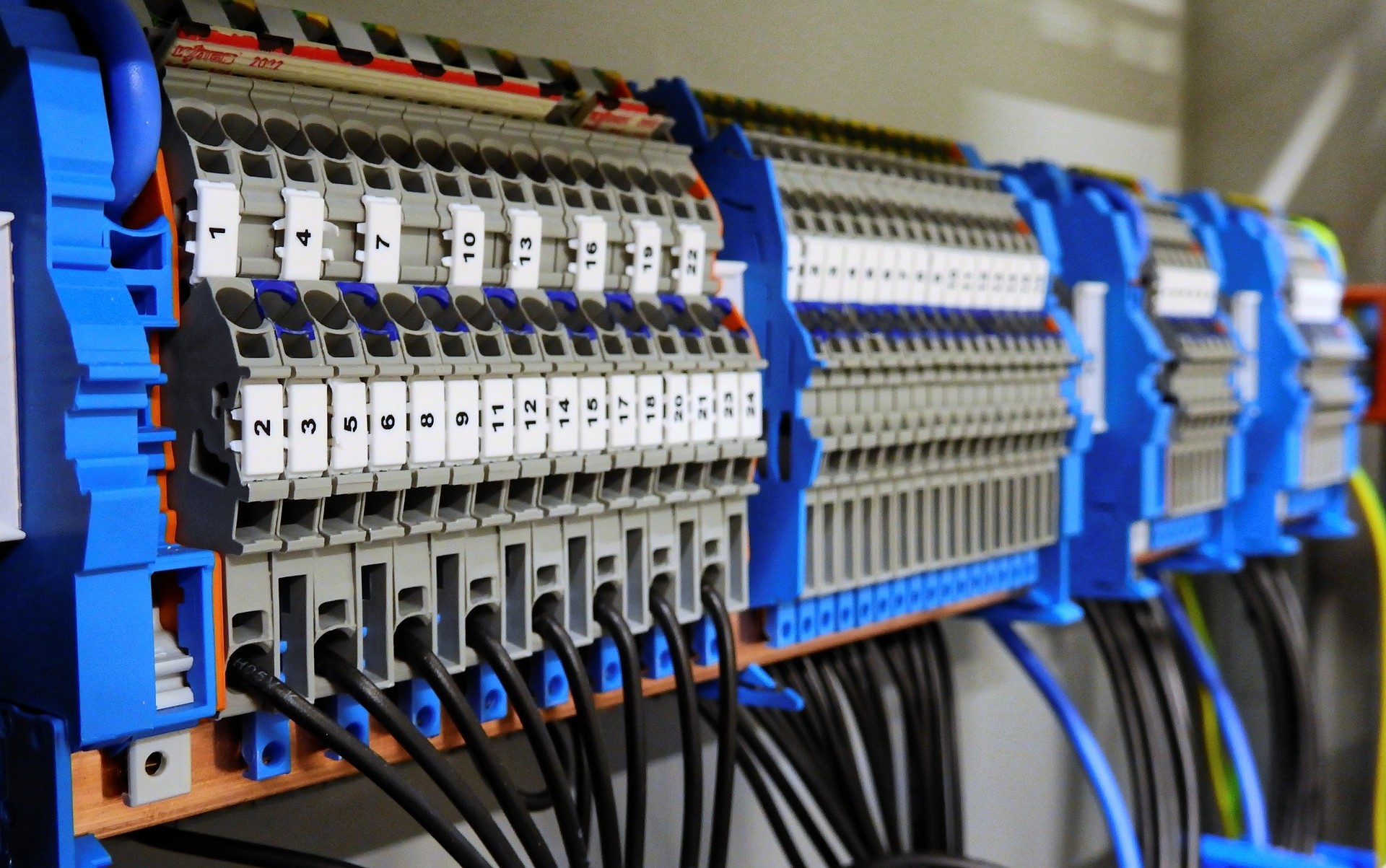

Compliance with regulations and procedures
Our deep understanding of regulations and safety procedures enables us to ensure a safe working environment that complies with pharmaceutical industry regulations.
We have a proven track record that allows us to manage complex procedures and requirements in the pharmaceutical sector with ease and skill, simplifying the path for our clients.
Design and commissioning of MT and BT systems
Support for GMP qualification and validation procedures
Design and commissioning of safety and security systems
Commissioning, Qualification and Validation (CQV)
The "Commissioning, Qualification, and Validation" (CQV) process is of utmost importance in the pharmaceutical field as it ensures that facilities and processes are adequately designed, verified, and validated to produce drugs safely, effectively, and in compliance with GMP rules.
Compliance with stringent regulations is crucial because any deviation can pose risks to patient health and product quality.
We work closely with companies in the pharmaceutical sector to ensure that their facilities and processes are precisely commissioned, qualified, and validated, strictly adhering to regulations and ensuring the production of high-quality and safe medications for patients.


Good Manufacturing Practices (GMP)
Our expertise extends to the precise implementation of processes, systems, and controls that scrupulously adhere to Good Manufacturing Practices (GMP).
We work diligently to ensure that electrical systems are impeccably designed and managed in line with strict GMP regulations. This includes the implementation ofadvanced control systems to ensure the safety, reliability, and efficiency of the facilities.
Our mission is to ensure that electrical systems are a solid cornerstone of pharmaceutical production, contributing to the quality and safety of the final products.
Efficiency and safety
Not only is the efficiency of the facility important, but safety is a fundamental factor.
The design of a new pharmaceutical facility must prioritize the health and well-being of the personnel who will be working in it.
Prevention and protection from injuries, accidents, and health hazards are paramount.


Respect for the environment
Environmental impact must be kept to an absolute minimum, and this is another critical factor to consider.
It's not just about keeping those who work in the facility safe, but also ensuring the safety and well-being of the surrounding environment and its inhabitants. Prevention and protection from potential injuries and health hazards extend not only to personnel but also to environmental preservation.
We design innovative and customized
electrical systems for pharmaceutical facilities,
with our primary focuses being
GMP quality, safety, efficiency, and the environment.
Our added values
Specialized expertise
Every industry demands a unique approach and specific knowledge. Our experience in designing for pharmaceutical facilities allows us to fully grasp the sector's needs and challenges, ensuring suitable and customized solutions.
Focus on quality
With our attention to detail and dedication, we are committed to ensuring that every project meets the highest quality standards, enabling pharmaceutical companies to achieve reliable and excellent results in line with GMP standards.
Safety and Compliance
Safety in the pharmaceutical sector is crucial. Our meticulous approach to project design and evaluation is based on adhering to all the necessary regulations and procedures to ensure a safe and compliant working environment.
Tailored design
Our ability to provide customized solutions is one of our greatest strengths. We work closely with clients to understand their specific needs and develop tailor-made projects that precisely meet their requirements, ensuring optimal results.
Integrated vision
Our years of experience enable us to have a comprehensive view of every project. In addition to considering the client's needs, we also take into account environmental impact and safety to create sustainable, long-term solutions
Reliability and punctuality
Our reputation for reliability and punctuality in project delivery makes us a valuable partner. Our clients can rely on us to meet established deadlines and deliver high-quality results promptly.
Ongoing support
Our commitment to the customer extends beyond the design and evaluation phase. We are always ready to provide continuous assistance, supporting clients during the commissioning of facilities and throughout the entire project lifecycle.
Sinergo Enterprise's extensive experience
is at the service of the client
in every phase of the project.
Our services
With a continuous commitment to ensuring quality, safety, efficiency, and environmental sustainability, we offer a range of specialized services to meet the diverse needs of the pharmaceutical sector.
- Electrical system design (medium/low voltage, safety, and security systems)
- Instrumental design
- Evaluation and validation of projects according to User Requirement Specifications (URS)
- Commissioning assistance
Want to learn more? Contact us.
Please fill out the form, you will be contacted as soon as possible.